Accusil tubing
Tubing for accurate filling with excellent dispensing accuracy ± 0.5 %. Eight sizes available.
Single-use assemblies
Call +971 4886 8840
CellefillTM is a turnkey, small batch, vial filling machine with an integrated containment solution. This system has been designed by aseptic fill/finish experts Flexicon Liquid Filling and containment experts Franz Ziel.
As a fully integrated solution for the biopharmaceutical industry, Cellefill maximises production capabilities through recipe driven operation and delivers enhanced levels of process assurance to accelerate your time to market.
Cellefill optimises your batch security with highly accurate peristaltic pump performance and integrated environmental monitoring.
Our vial filling machines with integrated containment solutions maximise production of advanced therapy medicinal products (ATMPs) including cell and gene therapies (CGT), small batch pharmaceuticals and biologics.
| Filling volume range | Limited only by RTU vial size, filling volume range is from micro-volumes to 50 ml |
| Accuracy | achievable at most volumes ±1.0 % |
| Capacity | 1500 units/hour |
| Stoppers | ISO standard or Lyo, 13 mm and 20 mm |
| Cap diameter range | ISO standard or flip-top, 13 mm or 20 mm |
| Infeed and outfeed trays | Vial RTU compatible with SG EZ-Fill® and SCHOTT adaptiQ® trays |
Technical Summary table lists features available within the range. See ‘Specifications’ tab for model specific information.
| Cellefill oRAB | Cellefill iASP | Cellefill iBIO | |
|---|---|---|---|
| Filling volume range | Limited only by RTU vial size, filling volume range is from micro-volumes to 50 ml | Limited only by RTU vial size, filling volume range is from micro-volumes to 50 ml | Limited only by RTU vial size, filling volume range is from micro-volumes to 50 ml |
| Accuracy | achievable at most volumes ±1.0 % | achievable at most volumes ±1.0 % | achievable at most volumes ±1.0 % |
| Capacity | 1500 units/hour | 1500 units/hour | 1500 units/hour |
| Batch Size | 10 - 10,000 vials per batch | 10 - 10,000 vials per batch | 10 - 10,000 vials per batch |
| Power supply | 230/400 V 3 ph, 50 Hz | 230/400 V 3 ph, 50 Hz | 230/400 V 3 ph, 50 Hz |
| Weight | 2900 kg | 5200 kg | 6430 kg |
| Load per square meter | 605 Kg | 1085 Kg | 1090 Kg |
| Barrier Technology | Open Restricted Access Barrier | Double Wall Aseptic Isolator | Double Wall Aseptic Isolator |
| Filling Line Type | Aseptic process filling line with manual disinfection | Aseptic process filling line with automated VHP bio-decontamination | Aseptic-containment process filling line. Automated VHP bio-decontamination providing protection of sterile products with aerosol containment features and optional post-production VHP cycle for inactivation of live product/biologic residues. |
| Application | Sterile product protection of sensitive biologics, antibodies, cell protein or nucleic acid-based products | Sterile product protection of sensitive biologics, antibodies, cell protein or nucleic acid-based products | Hazardous biologics, individualised or live products requiring highest levels of cross contamination control or BSL2 containment. |
| Cleanroom Class | Grade B / ISO 7 cleanroom | Grade C / ISO 8 cleanroom | Grade C / ISO 8 cleanroom |
| Vial Size | ISO 2R - 50R | ISO 2R - 50R | ISO 2R - 50R |
| Weigh Check | 20-100 % | 20-100 % | 20-100 % |
| Stoppers | ISO standard or Lyo, 13 mm and 20 mm | ISO standard or Lyo, 13 mm and 20 mm | ISO standard or Lyo, 13 mm and 20 mm |
| Cap diameter range | ISO standard or flip-top, 13 mm or 20 mm | ISO standard or flip-top, 13 mm or 20 mm | ISO standard or flip-top, 13 mm or 20 mm |
| Infeed and outfeed trays | Vial RTU compatible with SG EZ-Fill® and SCHOTT adaptiQ® trays | Vial RTU compatible with SG EZ-Fill® and SCHOTT adaptiQ® trays | Vial RTU compatible with SG EZ-Fill® and SCHOTT adaptiQ® trays |
| Port type | Castus® alpha RTP 190 iCON, Sartorius Biosafe® 110 Monolever | Castus® alpha RTP 190 iCON, Sartorius Biosafe® 110 Monolever | Castus® alpha RTP 190 iCON, Sartorius Biosafe® 110 Monolever |
| Validation | Turnkey Validation FAT, SAT IQOQ | Turnkey Validation FAT, SAT IQOQ, PQ support for Isolator | Turnkey Validation FAT, SAT IQOQ, PQ support for Isolator |
| Description | oRAB |
|---|---|
| Minimum required operational space | 2000 mm |
| Footprint LOA | 4330 mm |
| Footprint WOA | 1800 mm |
| Height Overall | 2760 mm |
| Description | iASP |
|---|---|
| Minimum required operational space | 2000 mm |
| Footprint LOA | 4470 mm |
| Footprint WOA | 2400 mm |
| Assumed Height of Cleanroom | 2737 mm |
| Height Above Cleanroom | 2302 mm |
| Height Overall | 5039 mm |
| Description | iBIO |
|---|---|
| Minimum required operational space | 2000 mm |
| Footprint LOA | 5324 mm |
| Footprint WOA | 2558 mm |
| Assumed Height of Cleanroom | 2737 mm |
| Height Above Cleanroom | 2897 mm |
| Height Overall | 5634 mm |
Cellefill provides confidence in scale-up and repeatability of your operation by combining the benefits of the FPC60 filling machine with an integrated Franz Ziel containment solution and a No Touch Transfer (NTT) debagging system.
With quick reference icons and provision of key guidance throughout the process, the advanced Cellefill interface is designed for an uncomplicated user experience, without compromise.
Facilitating remote set-up for filling, the data management system is fully accessible from any web device with access to:
· Real-time supervisory oversight
· Batch data retrieval
· Recipe creation
Designed for future regulatory requirement, Cellefill is compliant with EU and US GMP requirements including:
· 21 CFR part 11
· EudraLex Vol 4
With a centralised critical warning alert system, unified airflow studies, and strategically placed environmental monitoring probes and settle plates, Cellefill’s integrated design facilitates streamline validation and qualification improving your speed to market.
No-Touch-Transfer (NTT) is a GMP compliant process for debagging Ready-to-Use (RTU) vials, enabling loading of vials into the aseptic core while maintaining sterile integrity. Compatible with Schott adaptiQ® and SG EZ-fill® trays.
| Module name | Module description |
|---|---|
No Touch Transfer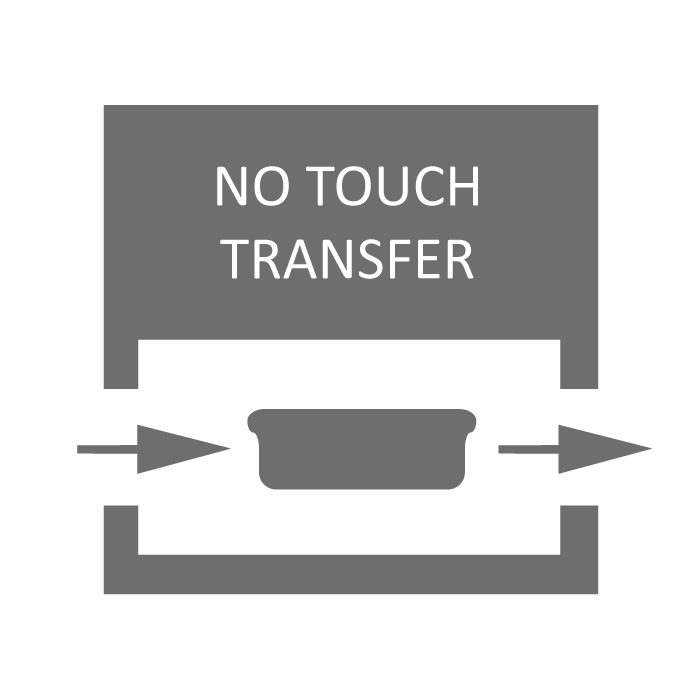 |
No-Touch-Transfer (NTT) is a GMP compliant process for debagging Ready-to-Use (RTU) vials, enabling loading of vials into asepctic core while maintaining sterile integrity There is an NTT process for de-bagging inner bags (oRAB model) or both inner/outer bags (iASP/iBIO models) |
Infeed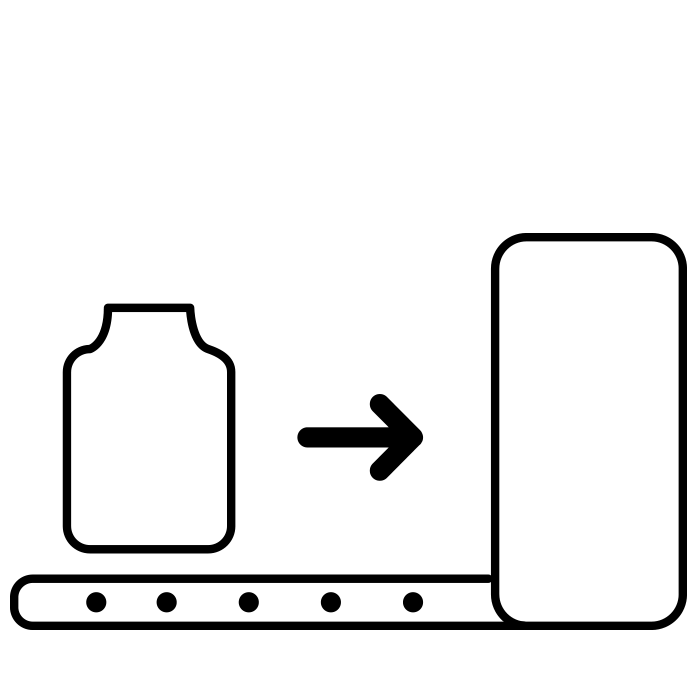 |
Tray turner for easy removal of vials from tray A buffering turntable aligns the vials and moves them to the starting position of the walking beam |
Filling |
Peristaltic pump solutions with easy operation and high accuracy Bottom up filling where filling nozzle raises along with fluid surface Vial position sensors, prevents mis-filling and contamination of the filling environment Up to 100 % in-line check weigh with zero waste start-up and in-batch dynamic calibration Gloveless operation in the aseptic core |
Stoppering |
Format parts: Chutes, jaws and pistons The universal vibrator bowl accommodates both ISO standard 13 mm and 20 mm injection and lyophilisation stoppers |
Capping |
Choice of crimp capping or roller closure, both with force closure measurement The Universal bowl accommodates both ISO standard 13 mm and 20 mm 'flip-top' and plain caps Format parts: Chutes and crimping heads. Rapid Transfer Ports and chutes for sterile transfer of stoppers and caps into the critical area |
Reject |
Automatic reject station transfers out of tolerance vials from the vial track down to the reject tray, based upon information relayed from any of the modules Collection of vials to be sampled intra-batch |
Outfeed |
Vials collected in trays or loaded robotically into baskets (iBIO version only) Finished vials can be removed without pausing production Options for Rapid Decontamination Station (RDS) for closed container surface decontamination (iBIO version only) |
 |
Strategically located environmental monitoring for critical risk assessment combination of viable/non-viable and active/passive probes. Environmental monitoring package standard in all configurations |
Tubing for accurate filling with excellent dispensing accuracy ± 0.5 %. Eight sizes available.
Single-use assemblies
Uniquely configurable fill/finish system. Innovative design with a wide variety of options to suit all requirements.
Aseptic filling - fully automatic systems
Filling and stoppering for small batch production. No costly overfilling – fill accuracy better than ±0.5 %.
Aseptic filling - fully automatic systems
Highly flexible aseptic filling system with integrated, full or partial stoppering and capping of vials.
Aseptic filling - fully automatic systems
Fully-automatic systems for production. Highly accurate filling of volumes from less than 0.2 ml to more than 250 ml.
Aseptic filling - fully automatic systems